Ionization Energy: Why Air Can Become a Conductor
Under normal conditions, air is almost a perfect dielectric. This is because its atoms and molecules are neutral and contain almost no free charges. However, when additional energy is introduced, air can become conductive.
How Air Conductivity Appears
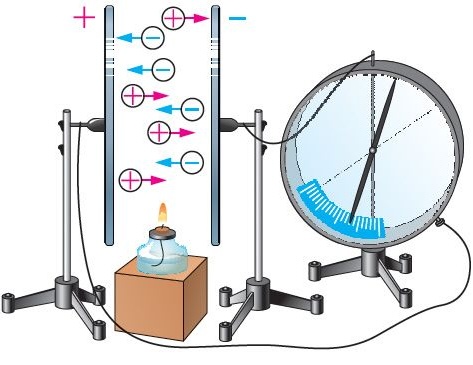
An experiment with an electrometer shows: at low voltage differences, air does not conduct electricity. But if the gap between electrodes is heated with a flame, the needle of the electrometer drops — an electric current begins to flow through the air. The reason is the emergence of free charge carriers (electrons and ions) caused by ionization. It is important to note that a flame acts not only as a heat source but also as a supplier of ionized particles, which drastically increase the air’s conductivity.What Is Ionization?
Ionization is the process of detaching electrons from atoms or molecules when a certain threshold energy, known as ionization energy, is reached. In an ionized gas, we observe:- positive ions (moving toward the cathode),
- free electrons,
- sometimes negative ions (formed when electrons attach to molecules).
Why Does the Current Stop?
When the flame is removed, electrons and ions recombine to form neutral molecules again. This stops the current. To maintain a discharge, continuous ionization is required — via heating, UV, or X-ray radiation.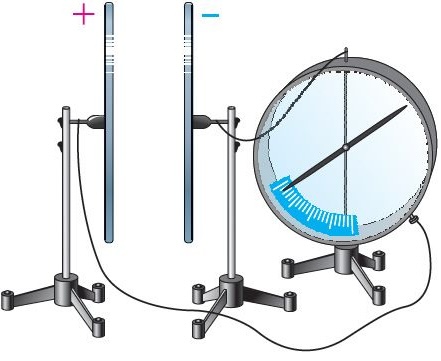
Volt-Ampere Characteristic of Gas Discharge: Physical Principles and Practical Applications
Fundamentals of Gas Discharge and Its Study
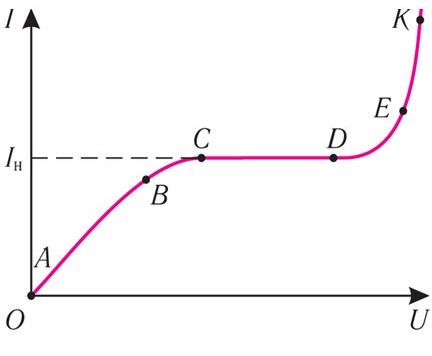
To analyze the physical processes of gas discharge in laboratory practice, a glass tube with two electrodes is used. The central focus of the study is the volt-ampere characteristic (VAC) — the dependence of current in the gas medium on the voltage applied to the electrodes.Mechanism of Gas Discharge Formation
When an external ionizer acts on the gas, ionization occurs in the inter-electrode space. This process is accompanied by the opposite phenomenon — recombination of ions into neutral atoms and molecules.Ohmic Region of the Characteristic
At the initial stage, with low voltages between the electrodes, a linear dependence of current on voltage is observed (segment A–B on the VAC). In this mode:- Only a small fraction of the generated ions and electrons reach the electrodes,
- Most of the charged particles recombine before reaching the electrodes,
- Ohm’s law is valid for the gaseous medium,
- The current is directly proportional to the applied voltage.
Transition Regime and Saturation Current
As the voltage increases further, the linear relationship breaks down (segment B–C). The saturation current (segment C–D) is reached when all charge carriers generated by the external ionizer reach the electrodes without recombination. In this regime, the current remains constant and no longer depends on an increase in voltage.Impact Ionization and Avalanche Process
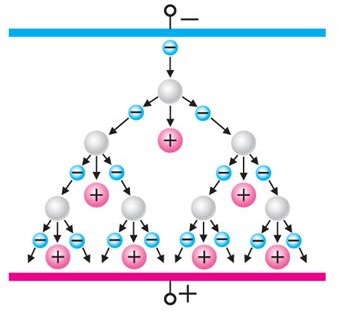
When a critical voltage is reached, free electrons gain enough kinetic energy to ionize atoms by impact. This process includes:- Acceleration of electrons in the electric field,
- Detachment of electrons from atoms during collisions,
- Formation of secondary ionizers,
- Avalanche-like increase in the number of charged particles.
Self-Sustaining Gas Discharge
Conditions for Occurrence
A self-sustaining discharge can continue without an external ionizer due to its own processes of charge carrier generation:- Secondary electron emission — the release of electrons from the cathode surface when bombarded by positive ions,
- Thermionic emission — the emission of electrons from a heated cathode surface.
Classification of Self-Sustaining Discharges
Glow Discharge
Characteristics:- Current: tens of milliamperes,
- Voltage: tens to hundreds of volts,
- Pressure: fractions of a millimeter of mercury.
- Gas-discharge tubes for advertising and decorative lighting,
- Fluorescent lamps,
- Neon light sources.
Arc Discharge
Characteristics:- Current: tens to hundreds of amperes,
- Voltage: tens of volts,
- Bright glow of the gas column.
- Powerful lighting systems,
- Arc welding and metal cutting,
- Electrolysis of melts,
- Industrial electric furnaces.
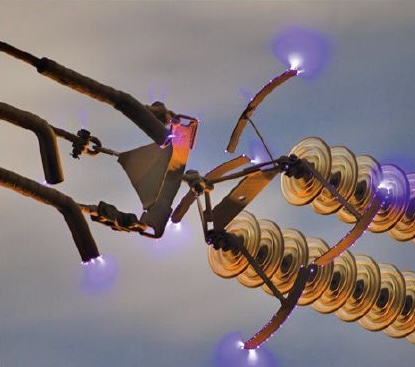
Corona Discharge
Conditions:- Atmospheric pressure,
- Strongly non-uniform electric field,
- Sharp conductor geometry.
- Weak glow resembling a crown,
- Characteristic crackling sound,
- Localized near sharp edges.
- Electrostatic precipitators for industrial gas purification,
- Unwanted energy losses on high-voltage power lines.
Spark Discharge
Characteristics:- High breakdown voltage,
- Bright and intense glow,
- Acoustic effects from sudden air pressure increase.
- Voltage: 10⁸–10⁹ V
- Current: ~10⁵ A
- Duration: ~10⁻⁶ s
- Channel diameter: 10–20 cm



Plasma Physics: Fundamental Properties and Industrial Applications
Physicochemical processes occurring during high-energy thermal interactions with gaseous matter result in the emergence of a distinctive aggregate state of matter designated as plasma.Genesis of Plasma State Formation
Ionization Mechanisms
When subjected to sufficiently elevated temperatures, all materials undergo vaporization into the gaseous phase, followed by enhanced thermal ionization processes. This phenomenon is characterized by the dissociation of neutral gas molecules into their constituent atomic components, which subsequently transform into ionic species through various mechanistic pathways:- Thermal ionization manifests through vigorous collisional interactions between atomic and molecular species at elevated temperatures, occurring when the kinetic energy associated with thermal motion exceeds the binding energy maintaining electrons within atomic orbitals.
- Photoionization constitutes the formation process of ionic and electronic species under electromagnetic radiation influence, wherein photon energy surpasses the ionization potential of atomic constituents.
- Impact ionization by electron bombardment occurs during charged particle bombardment of gaseous media, representing the primary mechanism operative in electrical discharge phenomena.
Plasma Definition and Characteristics
Plasma constitutes a fully or partially ionized gaseous medium wherein the concentrations of positive and negative charge carriers demonstrate virtual equilibrium. This condition is mathematically expressed through the equivalence of average charge densities: ρ+ = |ρ−|. This material state exhibits quasi-neutrality — a fundamental property whereby the aggregate negative particle charge equals the total positive charge within sufficiently large volumes over extended temporal intervals.Plasma Classification Systems
Temperature-Based Categorization
Plasma formations are subdivided into two principal categories based on characteristic particle temperatures:- Low-temperature plasma (T < 10⁵ K) encompasses plasma states generated through various electrical discharge processes in gaseous media. This category includes:
- Plasma within gas-discharge illumination systems and fluorescent light sources
- Plasma in decorative applications and displays
- Medical plasma for therapeutic interventions
- High-temperature plasma (T > 10⁶ K) is exemplified by stellar plasma, where temperatures reach tens of millions of degrees. Stars represent massive concentrations of high-temperature plasma sustaining thermonuclear fusion reactions.
Ionization Degree Classification
Depending on the fraction of ionized atoms, plasma is distinguished as:- Partially ionized plasma with low ionization degrees (< 1%)
- Fully ionized plasma where all atomic species are stripped of electrons
Cosmic Plasma Distribution
The plasma state represents the most prevalent form of matter throughout the Universe, comprising approximately 95% of all visible material mass. Cosmic plasma permeates interstellar and intergalactic regions, with intergalactic concentrations averaging one particle per cubic meter.Interstellar Medium
The interstellar medium exhibits extremely low density with typical concentration values ranging from 0.1-1000 atoms per cubic centimeter. Average electron concentrations in the Milky Way’s interstellar medium approximate 0.037 cm⁻³. Voyager spacecraft data revealed interstellar plasma densities varying from 0.055 cm⁻³ to 0.13 cm⁻³ with increasing distance from the heliosphere.Terrestrial Plasma Environment
Earth’s upper atmospheric layer — the ionosphere — constitutes weakly ionized plasma. Ionization results from solar ultraviolet and X-ray radiation exposure, along with high-energy cosmic ray particles. The ionosphere comprises neutral atoms and quasi-neutral plasma mixtures, with charged particle concentrations varying from 10² to 10⁵ cm⁻³ depending on altitude and temporal factors. Solar wind represents a continuous plasma stream of solar origin propagating radially from the Sun at velocities of 300-1200 km/s. Near Earth’s orbit, solar wind proton densities approximate 6 cm⁻³ with electron temperatures reaching 4×10⁵ K during periods of enhanced solar activity.Physical Properties of Plasma
Electrical Conductivity
High electrical conductivity represents a fundamental plasma characteristic, attributed to free charged particle presence. Plasma conductivity increases proportionally with the ratio of ionized atoms to total atomic count. Fully ionized plasma approaches superconductor-level conductivity.Electromagnetic Field Interactions
Enhanced charged particle mobility enables strong plasma interactions with external electric and magnetic fields. This property is utilized for magnetic confinement of high-temperature plasma in tokamak-type thermonuclear devices.Practical Plasma Applications
Illumination Technologies
Low-temperature plasma finds extensive application in contemporary lighting systems:- Fluorescent lamps utilize plasma discharge in mercury vapor to generate ultraviolet radiation, subsequently converted to visible light through phosphor coatings.
- Sulfur plasma light sources provide solar-approximated emission spectra, with 75% visible light output and substantially reduced ultraviolet content compared to conventional sources.
- Decorative plasma lamps, invented by Nikola Tesla in 1894, consist of glass spheres containing high-frequency electrodes generating spectacular electrical discharges at 5-10 W power levels.
Industrial Applications
Plasma material processing includes:- Surface etching and modification in microelectronics fabrication
- Thin-film deposition and surface activation procedures
- Plasma welding and metal cutting using high-temperature plasma jets
- Industrial emission treatment and waste processing
- Medical sterilization and disinfection procedures
- Ozone generation for purification processes

